Are we close to cheaper cleaner methanol?
A newly discovered nickel-gallium catalyst can convert carbon dioxide into methanol leaving fewer by-products, say scientists.
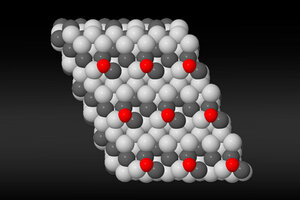
Artist's rendering of the nickel-gallium active site, which synthesizes hydrogen and carbon dioxide into methanol. Nickel atoms are light grey, gallium atoms are dark grey, and oxygen atoms are red.
Jens Hummelshoj/SLAC
We are probably a step closer to producing low-cost clean methanol, a potential source of fuel in the future.
Thanks to a newly discovered nickel-gallium catalyst that can convert hydrogen and carbon dioxide into methanol leaving behind considerably less carbon monoxide as a by-product, according to a paper titled "Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol" published in the March 2 online edition of the journal Nature Chemistry.
To start with, researchers spent three years studying and examining the copper-zinc-aluminum catalyst, a commonly used catalyst in the production of methanol.
Using a technique known as computational materials design, scientists did a threadbare analysis of hundreds of materials and compared them to the copper-zinc-aluminum catalyst.
"You get ideas for new functional materials based entirely on computer calculations. There is no trial-and-error in the lab first. You use your insight and enormous computer power to identify new and interesting materials, which can then be tested experimentally," Jens Nørskov, a professor of chemical engineering at Stanford and co-author said in a press release.
The team found that nickel-gallium catalyst would be probably the best one to replace the conventional copper-zinc-aluminum catalyst in the production of methanol.
After carrying out tests in the laboratory, the researchers confirmed that the new catalyst indeed produced less carbon monoxide and a higher yield of methanol. The temperature required for such a reaction is about 200-250 degree celsius, says Dr. Nørskov
"You also want a catalyst that's stable and doesn't decompose. The lab tests showed that nickel-gallium is, in fact, a very stable solid," said Ib Chorkendorff from the Technical University of Denmark and a co-author of the research paper.
But this new catalyst is not perfect yet, because it does leave some carbon monoxide behind.
In fact, if the nickel-gallium catalyst "contains just a few nanoparticles of pure nickel, the output drops quite a bit, because pure nickel is lousy at synthesizing methanol. In fact, it makes all sorts of chemical byproducts that you don't want," Dr. Chorkendorff said.
Nickel is plenty in nature and gallium is used in electronic industry.
Used in manufacturing paints, polymers, glues, and biofuels, methanol is "processed in huge factories at very high pressures using hydrogen, carbon dioxide and carbon monoxide from natural gas," said Felix Studt, a staff scientist at SLAC National Accelerator Laboratory and the lead author of the paper.
Current production of methanol though is dependent upon hydrogen and carbon dioxide, largely obtained from hydrocarbons (mainly methane), says Nørskov.
The bigger aim therefore is to "synthesize methanol using hydrogen from renewable sources, such as water split by sunlight, and carbon dioxide captured from power plants and other industrial smokestacks," Nørskov said.

