Ebola fight enters new phase with vaccine trials in Africa
Mass testing of two potential Ebola vaccines is underway in Liberia's capital after regional success in limiting new cases. The disease has claimed nearly 9,000 lives in West Africa.
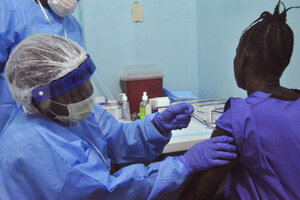
A woman is injected by a health care worker, left, as she takes part in an Ebola virus vaccine trial, at one of the largest hospital's Redemption hospital in Monrovia, Liberia, Monday, Feb. 2, 2015. A large-scale human trial of two potential Ebola vaccines got under way in Liberia's capital Monday, part of a global effort to prevent a repeat of the epidemic that has now claimed nearly 9,000 lives in West Africa.
Abbas Dulleh/AP
Researchers launched a large-scale human trial of two potential Ebola vaccines in Liberia on Monday, amid encouraging signs that the spread of the deadly virus in West Africa has slowed.
The vaccine trial comes as the three most affected countries – Liberia, Guinea, and Sierra Leone – have made considerable progress in preventing new infections. The World Health Organization (WHO) reported last week that the combined total of new cases in the three countries fell below 100 a week for the first time since June. (For comparison, Liberia alone experienced 300 new cases a week throughout August and September.)
As the epidemic enters what the WHO describes as a "second phase," the focus has shifted to ending the outbreak completely. More than 22,000 people have been infected, with nearly 9,000 lives lost, since the outbreak was first identified last spring. Provided the experimental vaccines prove effective on humans, health officials could use them to prevent any future Ebola outbreaks.
With 600 volunteers taking part in the first phase of the trial, scientists hope to learn whether they effectively prevent people from contracting the virus. Trial organizers say eventually as many as 27,000 people could take part, with a focus on immunizing front-line health workers.
Scientists are conducting the trial, which involves testing two vaccines created by two different drug companies, in the Liberian capital of Monrovia. They inject volunteers with a small amount of a strain of the Ebola virus to trick the body into producing an immune response, the BBC reports.
It’s a promising approach, but has stoked some concerns. As The Associated Press reports, “authorities still must combat fear and suspicion that people could become infected by taking part.”
Emmanuel Lansana, 43, a physician’s assistant, was the first to receive doses on Monday. Two shots were administered at different points on his right arm. His wife had expressed apprehension about the vaccine trial, but Lansana said he still wanted to take part.
“From the counseling, all of the reservations I have were explained, my doubts were cleared,” he said in a room where he was being observed for 30 minutes afterward.
As the vaccine trial gets underway, a separate team of researchers in Monrovia has halted a clinical trial of a drug to treat people infected with Ebola because of the sharp decline in patients. Chimerix, the developer of the antiviral drug, made the announcement Friday.
“Without having enough patients there to make any conclusions, it wasn’t feasible for us to push forward,” Dr. M. Michelle Berrey, chief executive of Chimerix, told The New York Times.
Chimerix shipped enough of the drug to Liberia for 140 patients, but it has treated fewer than 10 since the trial started on Jan. 2.
A separate antiviral drug study is being carried out in Guinea. But organizers of that trial are now looking for another clinic in order to find more patients, the Times reports. While a declining number of new Ebola patients could also affect other planned trials, researchers say it’s a good problem to have.
“It’s more important to end the outbreak than to get the trial done,” Dr. Armand Sprecher, a public health specialist for Doctors Without Borders, told the Times.

